Carbon Steel in Seawater 2
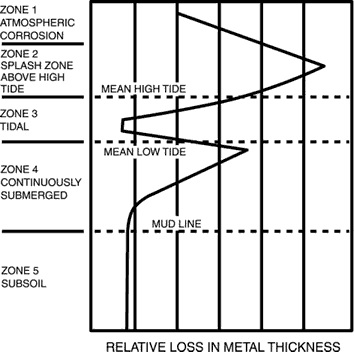
The corrosion rate of carbon steel in quiescent seawater is ~0.1mm/y, but the rate can vary markedly with position, relative to the mean high and low tide level (see below). Protection of a steel structure can be achieved by various means. Each corrosion zone shown below must be separately considered. Generally accepted methods to control corrosion are cathodic protection (CP), painting or coating, and sheathing.
The spray and splash zone above the mean high-tide level is the most severely attacked region because of continuous contact with highly aerated seawater, and the erosive effects of spray, waves, and tidal actions. Corrosion rates as high as 0.9 mm/y at Cook Inlet, Alaska, and 1.4 mm/y in the Gulf of Mexico have been observed. Cathodic protection in these zones is ineffective because of the lack of continuous contact with the seawater (the electrolyte), and thus no current flows between the metals for much of the time. Coatings need to be very robust as the conditions can be damaging. Sheathing in the forms of neoprene, other rubber coatings or nickel and copper alloys have been used successfully.
Corrosion rates for bare steel pilings, etc., also can be high at the position just below mean low tide and are caused by galvanic effects because of the different levels of aeration that occur in the tidal region. Corrosion can be controlled by CP systems because the area is continuously immersed.
Corrosion just above and below the mud line are both about 0.1mm/y, because the action of sulphate reducing bacteria in the mud provides analternative cathodic reaction to the reduction of dissolved oxygen, which is the cathodic reaction above the mud line.
Posted on: 21st July 2017

